Awe-Inspiring Examples Of Tips About How To Draw Lewis Structures For Molecules
(for example, h 2 o has 2x1 + 6 = 8 valence electrons, ccl 4 has 4 + 4x7 = 32.
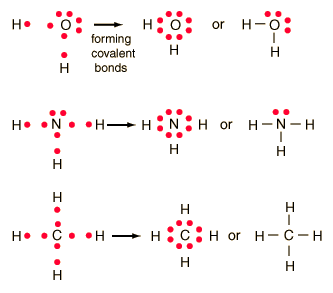
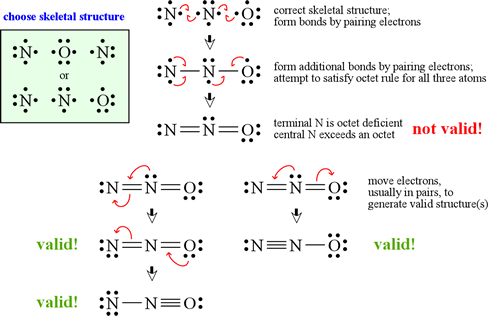
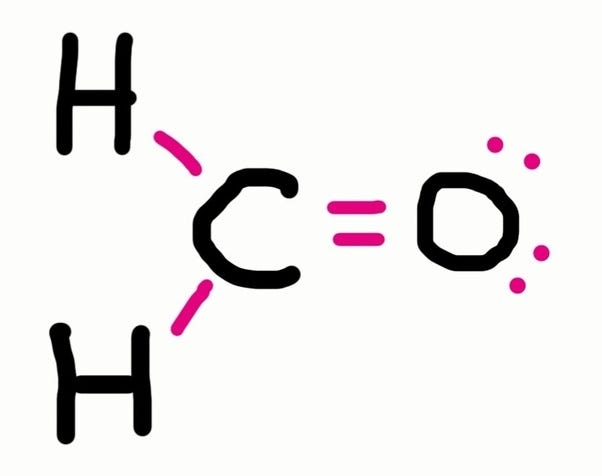
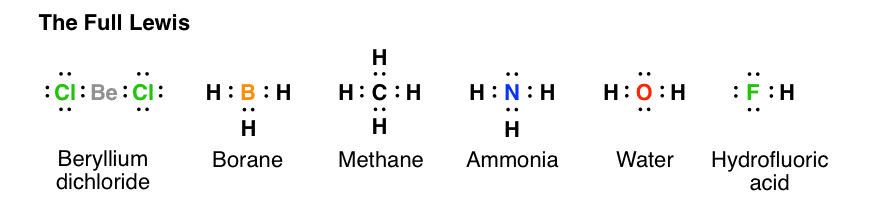
How to draw lewis structures for molecules. The lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom. Rules for writing lewis structures. Write the correct skeletal structure for the molecule.
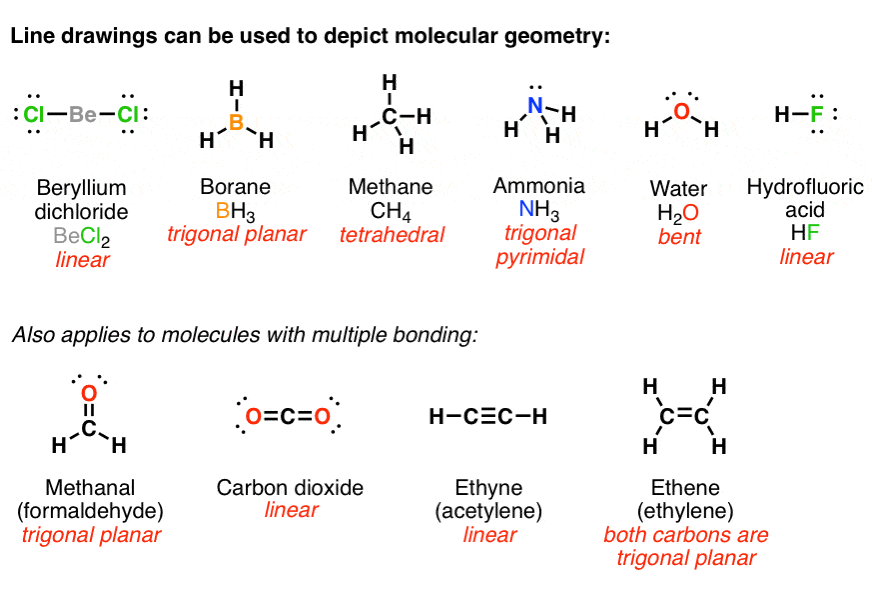
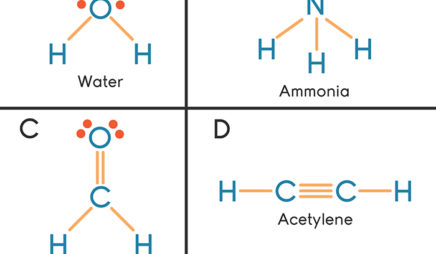
This can be determined by looking at the group number at the top of the column. First of all, a correct count of all. Figure 1.2a the lewis structures of aluminum, tin, nitrogen, chlorine and bromine.
In this way, 8 valence electrons. How to draw lewis structures for molecules that contain no charged atoms. In drawing lewis structures for relatively small molecules and polyatomic ions, the structures tend to be more stable when they are compact and symmetrical rather than extended chains of.
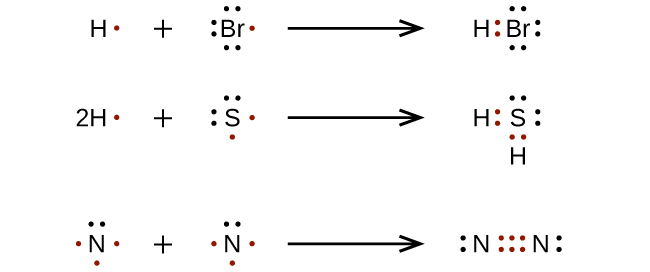
When we draw the lewis dot structure of ammonium ion, we subtract the one valence electron from the total number of valence electrons. Rules for drawing lewis structures. Steps to write lewis structures for diatomic molecules.
Click on the second ring type in the ring menu to select it. To draw two fused rings: In this lab you will draw lewis structures for a number of.
To learn more about this topic and other related topics, register with. To do this, find the number of valence electrons for each. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.


/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)






:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png)

